Followers
Saturday, June 27, 2009
MID YEAR RESULT
Albert Tay 59
Andy Malvine 65
Ang Fong Ying 82
Aurelia Tay 47
Beatrice Selester 54
Cecelia Simon 48
Charleston Chin 59
Christopher Primus 47
Cliff Rayner Azmi 48
Cynthia Hilarius 80
Esra Daniel 57
Eva Brenda Philip 38
Gerard Fung 76
Iresh Chia 71
Jessel Daniel 34
Jessie Daud 78
Kenneth Yong 62
Mohd Afiq Iqbal 78
Mohd Asraf Ruzaini 52
Natalie Chu 63
Nurhidayah 35
Nurul Suhaila Jamal 61
Ong Min Li 55
Sharizah Warno 59
Simon Chung 60
Stelly Lai 53
Vera Gan 61
Electroplating..
What is electroplating?
Electroplating is the deposition of a metallic coating by putting a negative charge on an object and exposing it to a solution containing a metal salt. The positively charged metal ions in the salt solution are attracted to the object and reduced to metallic form upon it.
How does it work?
Look at the figure above: We have a metallic object we want to plate with a metal. First we fill a cell with a solution of a salt of the metal to be plated. Most of the time the salt (nickel chloride in our example) is simply dissolved in water and a little acid.
The NiCl2 salt ionizes in water into Ni++ ions and two parts of Cl- ions.
A wire is attached to the object, and the other end of the wire is attached to the negative pole of a battery (with the blue wire in this picture) and the object is immersed in the cell. A rod made of nickel is connected to the positive pole of the battery with the red wire and immersed in the cell.
Because the object to be plated is negatively charged (by being connected to the negative pole of the battery), it attracts the positively charged Ni++ ions. These Ni++ ions reach the object, and electrons flow from the object to the Ni++ ions. For each ion of Ni++, 2 electrons are required to neutralize its positive charge and 'reduce' it to a metallic atom of Ni0. Thus the amount of metal that electroplates is directly proportional to the number of electrons that the battery provides.
This relationship is a reflection of Faraday's Law of Electrolysis. If you are advanced enough in chemistry (a high school student), that you've heard terms like gram molecular weight, mole, valence and Avagadro's number, but it's all a hodepodge to you instead of a cohesive whole, don't despair! Study Faraday's Law, and suddenly all of these disparate wacky terms will come together in a moment of enlightenment.
Meanwhile back at the anode, electrons are being removed from the Nickel metal, oxidizing it to the Ni++ state. Thus the nickel anode metal dissolves as Ni++ into the solution, supplying replacement nickel for that which has been plated out, and we retain a solution of nickel chloride in the cell.
As long as the battery doesn't go dead, nickel continues to dissolve from the anode and plate out onto the cathode.
We used nickel chloride in the example chiefly for simplicity of explanation. First, because nickel always dissolves in the "+2" oxidation state (Ni++), whereas many other metals like copper and zinc can dissolve in either the "+1" or "+2" state and add some confusion; secondly because chloride is a simple one-atom anion whereas most anions like sulphate or acetate are far more complex. But we do not recommend that nickel be used for school science demonstrations because -- while the explaining is simple -- the plating is difficult :-)
For school demonstrations, we suggest plating copper pennies with zinc, or plating quarters or brass keys with copper.
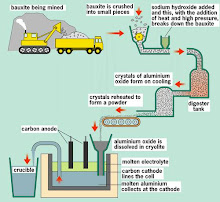
Purification...
Purification of copper
When copper is made from sulphide ores by the first method above, it is impure. The blister copper is first treated to remove any remaining sulphur (trapped as bubbles of sulphur dioxide in the copper - hence "blister copper") and then cast into anodes for refining using electrolysis.
Electrolytic refining
The purification uses an electrolyte of copper(II) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes.
The diagram shows a very simplified view of a cell.
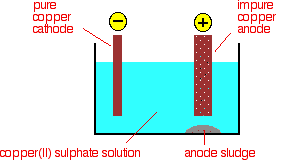
At the cathode, copper(II) ions are deposited as copper.
![]()
![]()
At the anode, copper goes into solution as copper(II) ions.
![]()
![]()
For every copper ion that is deposited at the cathode, in principle another one goes into solution at the anode. The concentration of the solution should stay the same.
All that happens is that there is a transfer of copper from the anode to the cathode. The cathode gets bigger as more and more pure copper is deposited; the anode gradually disappears.
In practice, it isn't quite as simple as that because of the impurities involved.
What happens to the impurities?
Any metal in the impure anode which is below copper in the electrochemical series (reactivity series) doesn't go into solution as ions. It stays as a metal and falls to the bottom of the cell as an "anode sludge" together with any unreactive material left over from the ore. The anode sludge will contain valuable metals such as silver and gold.
Metals above copper in the electrochemical series (like zinc) will form ions at the anode and go into solution. However, they won't get discharged at the cathode provided their concentration doesn't get too high.
The concentration of ions like zinc will increase with time, and the concentration of the copper(II) ions in the solution will fall. For every zinc ion going into solution there will obviously be one fewer copper ion formed. (See the next note if you aren't sure about this.)
The copper(II) sulphate solution has to be continuously purified to make up for this.
Monday, June 15, 2009
electrochemistry...
In this topic, you'll have to be able to learn about electrolytes, half equation, factors affecting electrolysis (Electrochemiscal Series, Type of electrode and Concentration of electrolyte), electrolysis in industry (electroplating, extraction, purification), voltaic cell....
Electrochemical Series (ES) for positive ions
K (Kalau)
Na (Nak)
Ca (Cari)
Mg (Makan)
Al (Ambil)
Zn (Zat)
Fe (Besi)
Sn (Supaya)
Pb (Penyakit)
Hg (Hilang)
Cu (Cepat)
Ag (Ahhh)
ES for negative ions
F (Fikir)
SO4 (Sebelum)
NO3 (Nak)
CO3 (Cari)
B (Bakal)
I (Isteri)
O (Ok)