Purification of copper
When copper is made from sulphide ores by the first method above, it is impure. The blister copper is first treated to remove any remaining sulphur (trapped as bubbles of sulphur dioxide in the copper - hence "blister copper") and then cast into anodes for refining using electrolysis.
Electrolytic refining
The purification uses an electrolyte of copper(II) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes.
The diagram shows a very simplified view of a cell.
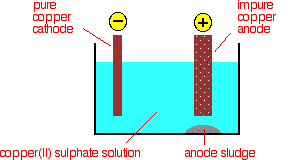
At the cathode, copper(II) ions are deposited as copper.
![]()
![]()
At the anode, copper goes into solution as copper(II) ions.
![]()
![]()
For every copper ion that is deposited at the cathode, in principle another one goes into solution at the anode. The concentration of the solution should stay the same.
All that happens is that there is a transfer of copper from the anode to the cathode. The cathode gets bigger as more and more pure copper is deposited; the anode gradually disappears.
In practice, it isn't quite as simple as that because of the impurities involved.
What happens to the impurities?
Any metal in the impure anode which is below copper in the electrochemical series (reactivity series) doesn't go into solution as ions. It stays as a metal and falls to the bottom of the cell as an "anode sludge" together with any unreactive material left over from the ore. The anode sludge will contain valuable metals such as silver and gold.
Metals above copper in the electrochemical series (like zinc) will form ions at the anode and go into solution. However, they won't get discharged at the cathode provided their concentration doesn't get too high.
The concentration of ions like zinc will increase with time, and the concentration of the copper(II) ions in the solution will fall. For every zinc ion going into solution there will obviously be one fewer copper ion formed. (See the next note if you aren't sure about this.)
The copper(II) sulphate solution has to be continuously purified to make up for this.






Apna Showroom Plastic Hair Clutcher for Women hair clips for girls (Combo of 12 Pieces Multicolour, small)
ReplyDeletemoney chumbak financail planner investment retirement specialist gurgaon gurugram ncr delhi panipat,
Apna Showroom Baby Girls' Plastic Hair Clips Claw Clutcher (Multicolour)-35 Pieces Girls/women
yeah very true meet me and i will come to you