




This is a specially created blog for science stream students' of SMK Chung Hwa Tenom. We aim to get students discussing their opinions on chemistry and also to share extra info when chemistry is concerned. Ex-students are welcome to participate. Let's show everyone, V Tenom people are also technology savvy!!!
| Color of Gold | Alloy Composition |
| Yellow Gold (22K) | Gold 91.67% Silver 5% Copper 2% Zinc 1.33% |
| Red Gold (18K) | Gold 75% Copper 25% |
| Rose Gold (18K) | Gold 75% Copper 22.25% Silver 2.75% |
| Pink Gold (18K) | Gold 75% Copper 20% Silver 5% |
| White Gold (18K) | Gold 75% Platinum or Palladium 25% |
| White Gold (18K) | Gold 75% Palladium 10% Nickel 10% Zinc 5% |
| Gray-White Gold (18K) | Gold 75% Iron 17% Copper 8% |
| Soft Green Gold (18K) | Gold 75% Silver 25% |
| Light Green Gold (18K) | Gold 75% Copper 23% Cadmium 2% |
| Green Gold (18K) | Gold 75% Silver 20% Copper 5% |
| Deep Green Gold (18K) | Gold 75% Silver 15% Copper 6% Cadmium 4% |
| Blue-White or Blue Gold (18K) | Gold 75% Iron 25% |
| Purple Gold | Gold 80% Aluminum 20% |

In order for a double-replacement reaction to take place, ion has to encounter ion. This typically takes place in solutions. Furthermore, one of the products must be either a molecule, like water, or a gas or an insoluble salt. The following salts, which are found on the back of this sheet, are insoluble. This means that they do not dissolve in water. Your task is to choose to make one of these salts and to separate it in a pure form, using the solubility properties and double replacement reactions. Furthermore, each of the salts has a challenge associated with it. With the help of your instructor your group will choose a salt to make. (Only one group per salt.) A list of starting salts from which you can choose and make your own insoluble salt is at the top of the backside of this sheet. Over the course of the next week you will complete this project and hand in your report as a chemical journal article. (Sample journal articles are available in class for your perusal.)
In your Journal Article you will need to give an account of what you have done. This article will need to contain:
(a) an introductory paragraph in which you lay out your overall purpose, outline a basic procedure, and explain key concepts like a double replacement reaction. Be sure to be specific in your introduction about the salt you are making. Include specific chemical equation(s).
(b) a list your materials and equipment used.
(c) a step by step procedure so that someone else could repeat the lab you did.
(d) recorded measurements and observations. (Be sure to include any measured values, such as masses or volumes or times.)
(e) a explanation of how you met (or tried to meet) the challenge. Put this in a paragraph called “Meeting the Challenge.”
(f) a summary of your overall results within some concluding remarks.
Guidelines for Procedure:
Use small amounts of the salts (i.e. <>
Be careful not to contaminate the salt supplies; use what you need, put the top back on and return the salt container to the centralized tray.
Work in the smaller test tubes so that they will fit into the centrifuge.
When using the centrifuge be sure that it is always counterbalanced on the opposite side with a test tube of water of equal volume.
Record what you do as you do it. Don’t worry about back tracking and trying again.
Dispose of waste solutions and salts in an appropriate waste container as provided.
The next page contains the salts from which you can choose the one you would like. Discuss with your instructor which one would be right for your lab group to choose.
Available Salts:
Sodium carbonate potassium hydroxide cobalt (II) chloride
Zinc sulfate nickel (II) sulfate nickel (II) chloride
Aluminum chloride magnesium sulfate calcium chloride
Copper (II) sulfate copper (II) chloride sodium hydroxide
Magnesium chloride
SALTS TO MAKE – Choose one to make and meet the challenge.
1. Make: Nickel (II) carbonate
Challenge: Take the nickel carbonate, add HCl, interpret results
2. Make: Zinc carbonate
Challenge: After you have the zinc carbonate pure salt, find a way to dissolve it.
3. Make: Cobalt (II) hydroxide
Challenge: Prove to your instructor that all the cobalt ions you started with have been converted to cobalt hydroxide.
4. Make: Aluminum hydroxide
Challenge: Find a way to turn aluminum hydroxide into aluminum nitrate
5. Make: Magnesium carbonate
Challenge: Test pH of all solutions you used. Interpret results.
6. Make: Calcium sulfate
Challenge: End with a blue layer in your test tube
7. Make: Copper (II) hydroxide
Challenge: Gently heat the copper hydroxide using a bunsen burner.
Make a hypothesis as to the identity of the "new" salt formed.
Electroplating is the deposition of a metallic coating by putting a negative charge on an object and exposing it to a solution containing a metal salt. The positively charged metal ions in the salt solution are attracted to the object and reduced to metallic form upon it.
Look at the figure above: We have a metallic object we want to plate with a metal. First we fill a cell with a solution of a salt of the metal to be plated. Most of the time the salt (nickel chloride in our example) is simply dissolved in water and a little acid.
The NiCl2 salt ionizes in water into Ni++ ions and two parts of Cl- ions.
A wire is attached to the object, and the other end of the wire is attached to the negative pole of a battery (with the blue wire in this picture) and the object is immersed in the cell. A rod made of nickel is connected to the positive pole of the battery with the red wire and immersed in the cell.
Because the object to be plated is negatively charged (by being connected to the negative pole of the battery), it attracts the positively charged Ni++ ions. These Ni++ ions reach the object, and electrons flow from the object to the Ni++ ions. For each ion of Ni++, 2 electrons are required to neutralize its positive charge and 'reduce' it to a metallic atom of Ni0. Thus the amount of metal that electroplates is directly proportional to the number of electrons that the battery provides.
This relationship is a reflection of Faraday's Law of Electrolysis. If you are advanced enough in chemistry (a high school student), that you've heard terms like gram molecular weight, mole, valence and Avagadro's number, but it's all a hodepodge to you instead of a cohesive whole, don't despair! Study Faraday's Law, and suddenly all of these disparate wacky terms will come together in a moment of enlightenment.
Meanwhile back at the anode, electrons are being removed from the Nickel metal, oxidizing it to the Ni++ state. Thus the nickel anode metal dissolves as Ni++ into the solution, supplying replacement nickel for that which has been plated out, and we retain a solution of nickel chloride in the cell.
As long as the battery doesn't go dead, nickel continues to dissolve from the anode and plate out onto the cathode.
We used nickel chloride in the example chiefly for simplicity of explanation. First, because nickel always dissolves in the "+2" oxidation state (Ni++), whereas many other metals like copper and zinc can dissolve in either the "+1" or "+2" state and add some confusion; secondly because chloride is a simple one-atom anion whereas most anions like sulphate or acetate are far more complex. But we do not recommend that nickel be used for school science demonstrations because -- while the explaining is simple -- the plating is difficult :-)
For school demonstrations, we suggest plating copper pennies with zinc, or plating quarters or brass keys with copper.
Purification of copper
When copper is made from sulphide ores by the first method above, it is impure. The blister copper is first treated to remove any remaining sulphur (trapped as bubbles of sulphur dioxide in the copper - hence "blister copper") and then cast into anodes for refining using electrolysis.
Electrolytic refining
The purification uses an electrolyte of copper(II) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes.
The diagram shows a very simplified view of a cell.
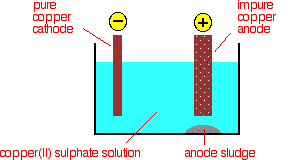
At the cathode, copper(II) ions are deposited as copper.
![]()
![]()
At the anode, copper goes into solution as copper(II) ions.
![]()
![]()
For every copper ion that is deposited at the cathode, in principle another one goes into solution at the anode. The concentration of the solution should stay the same.
All that happens is that there is a transfer of copper from the anode to the cathode. The cathode gets bigger as more and more pure copper is deposited; the anode gradually disappears.
In practice, it isn't quite as simple as that because of the impurities involved.
What happens to the impurities?
Any metal in the impure anode which is below copper in the electrochemical series (reactivity series) doesn't go into solution as ions. It stays as a metal and falls to the bottom of the cell as an "anode sludge" together with any unreactive material left over from the ore. The anode sludge will contain valuable metals such as silver and gold.
Metals above copper in the electrochemical series (like zinc) will form ions at the anode and go into solution. However, they won't get discharged at the cathode provided their concentration doesn't get too high.
The concentration of ions like zinc will increase with time, and the concentration of the copper(II) ions in the solution will fall. For every zinc ion going into solution there will obviously be one fewer copper ion formed. (See the next note if you aren't sure about this.)
The copper(II) sulphate solution has to be continuously purified to make up for this.

Lithium is a Group 1 element containing just a single valence electron. Group 1 elements are called "alkali metals". Lithium is a solid only about half as dense as water and lithium metal is the least dense metal. A freshly cut chunk of lithium is silvery, but tarnishes in a minute or so in air to give a grey surface. Its chemistry is dominated by its tendency to lose an electron to form Li+. It is the first element within the second period. Lithium is mixed (alloyed) with aluminium and magnesium for light-weight alloys, and is also used in batteries, some greases, some glasses, and in medicine.